
Tumours do not exist as isolated entities but are embedded within their ‘microenvironment,’ the cells and tissue which surround it. Tumours and their microenvironment interact and influence each other and so it is important, when we think about new ways to treat cancer, that we also think about the microenvironment.
The immune system protects us by recognising and attacking things which might make us ill, such as viruses, bacteria, or unhealthy cells. Immunotherapy aims to train the body’s own immune system to become better at finding and destroying tumour cells. However, so far, this approach has had limited success in prostate cancer. One reason might be that the prostate cancer microenvironment is good at shutting down immune system components. This team of PCRC researchers wanted to investigate if they could overcome this issue by administering their immunotherapy directly into the tumour.
A number of different approaches are being tried to make immunotherapy work in prostate cancer.
This research team are particularly interested in a protein called interleukin-15, or IL-15. IL-15 is known to be important in the immune system, and the team discovered that when it was in the presence of prostate cancer cells, it could both activate and increase the number of immune cells.
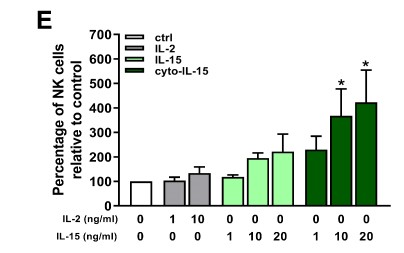

The researchers engineered IL-15 and two other drugs, antibodies called anti-CTLA-4 and anti-PD-L1, so that they could attach to cells. The results of this study proved, firstly, that the engineered drugs worked: they could attach to cells, and lab-based tests, in cells, showed that they could increase the population of a type of immune cell called Natural Killer (NK) cells, which play important roles in fighting both viruses and tumour cells. The engineered IL-15 also resulted in dramatic genetic changes in cancers in mice, affecting many genes normally involved in how cells move, and how they reproduce.
But the most exciting findings were that the engineered IL-15, both when it was used on its own and when it was used with the other two antibodies, not only slowed down the growth of tumours in mice by 52% (just engineered IL-15) and 58% (both engineered IL-15 and antibodies), mice treated with them also lived longer. The statistical analysis carried out on these results showed that they were most likely not due to chance, but were due to the treatments working. And, these mice didn’t appear to suffer any side effects.
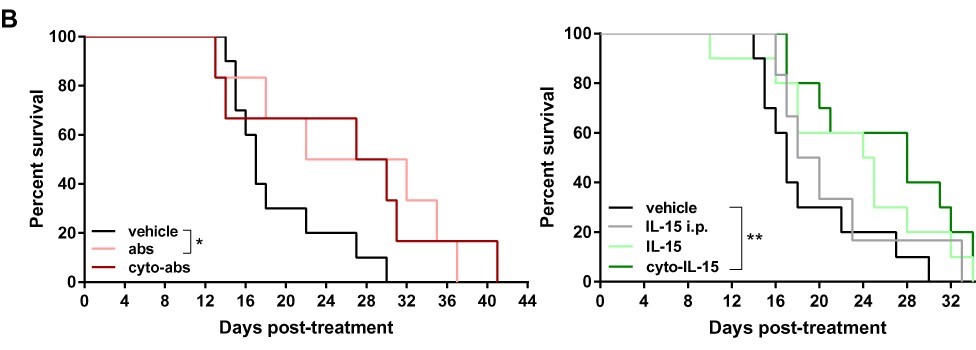

Some clinical trials have tried to use IL-15, for other cancers, by injecting it directly into the blood. The problem these trials have had is that the balance between the dose which can effectively treat cancers and the dose which causes serious side effects is extremely delicate. This study shows that, by injecting IL-15 directly into the prostate tumour, and anchoring it there, that a higher dose can be concentrated within the tumour, where it can be more effective, while side effects should be very limited.
This is a scientific study and so it will be some time before it reaches humans, but this paper shows that there is a strong scientific rationale to test this potential new therapy in clinical trials.
“We are pleased to confirm the publication of our work on our novel agent “tailed” or “cyto” IL-15. “Tailed” IL-15 is a sticky form of a naturally occurring protein, IL-15, present in minute amounts in the body. This protein stimulates the immune system to increase cells that can kill tumour cells. This study shows that our product, which can localise (stick) to the tissue it is injected into, can shrink tumours and increase survival of mice - when injected into prostate tumours more efficiently than its non-modified version. The publication of this work in a high impact peer reviewed journal validates our work, leading the way to further research with combinations of our agent with other agents and the possible development of the “tailed” IL-15 into a clinical grade product.
Dr Efthymia Papaevangelou and Dr Christine Galustian
Scientific publications, commonly referred to as papers, are how scientists share their own work or review the work done by others. In order to be accepted for publication in a journal, they must first be approved by experts in the field (“peer-reviewed”). As such, they are a testament to a scientific team’s credibility and the quality and reliability of their work. Both the stamp of approval given by a publication and the ability for other scientists to learn about and build upon a team’s research are important steps to take lab science closer to real-life benefits for humans.
This publication is an original research article, in which a team of scientists share their own work and discoveries.
This paper, under the full title “Targeting Prostate Cancer Using Intratumoral Cytotopically Modified Interleukin-15 Immunotherapy in a Syngenic Murine Model” was published in the journal ImmunoTargets and Therapy. The publication is open access, meaning the information is available to readers at no cost, and the journal’s Impact Factor (a measure of a journal’s influence in academic research circles) is 10.5. The top 2% of journals have an Impact Factor above 10.
The researchers who contributed to this paper are Efthymia Papaevangelou, Dorota Smolarek, Richard A Smith, Prokar Dasgupta, and Christine Galustian. They are based at King’s College, London, where this work was carried out. Prokar Dasgupta also works at the Urology Centre in Guy’s Hospital, London.
The researchers engineered IL-15 and two other drugs, attacking proteins called CTLA-4 and PD-L1, so that they could attach to cells. They explored the potential of these engineered drugs, which they called cyto-IL-15 and cyto-abs, as an immunotherapy both used alone and in combination (cyto-combo).
For the benefit of the scientific community who may wish to carry out similar experiments, the methods they used, including cell culture, cell proliferation assays, RNA sequencing, histology and immunofluorescence, and cytotopic protein modification, are described in the full paper. Prostate Cancer Research Centre adheres to the AMRC Statement on the Use of Animals in Research and animal experiments in this study were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986.